Welcome to Greener Concrete, LLC
What Are Zeolites?
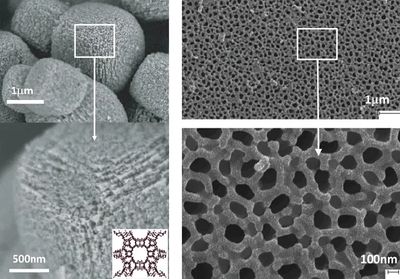
Zeolites are aluminosilicate minerals made from interlinked tetrahedra of alumina (AlO4) and silica (SiO4) with a relatively open, three-dimensional, honeycomb like crystal structure built from the elements aluminum, oxygen, and silicon, with alkali or alkaline-Earth metals (such as sodium, potassium, and magnesium) plus water molecules trapped in the gaps between them. Zeolites have open pores (sometimes referred to as cavities) in a very regular arrangement and roughly the same size as small molecules. Zeolite crystals have a Mohs hardness of 3.5 to 4, a typical pH > 9, and a specific gravity of 2.1 to 2.2.
There are about 40 naturally occurring zeolites, which are formed when layers of ash and rocks formed after volcanic eruption crystallize in post-depositional environments over periods ranging from thousands to millions of years. According to the US Geological Survey, the most commonly mined forms include chabazite, clinoptilolite, and mordenite. Our mines contain clinoptilolite zeolite, which is ideal for removal of heavy metals like Pb, Cd, Cu, Zn, Cr, Ni, Co, and Fe from contaminated water and soil. Zeolite is one of the rare minerals that possess a natural negative charge. The combination of the honeycomb structure and net negative charge allows Zeolite to both absorb liquids and adsorb elements based on the strength of the chemical bond.
ADsorption or Cation Exchange Capacity
Zeolite is one of the only negatively charged minerals in the world allowing it to be able to have what is called a “cation exchange capacity” or CEC. This CEC is the most outstanding property of zeolite making it an ideal detoxifying agent. CEC occurs when two or more positively charged compounds or elements exchange places on a negatively charged host such as zeolite. The most common exchangeable cations found in natural zeolite molecules are calcium, magnesium, potassium and sodium. The ability to release beneficial elements while capturing and binding elements that are detrimental to life makes zeolite an ideal mineral for the selective and intelligent adsorption of toxic elements.
ABsorption
Zeolite has an open framework molecular structure meaning it is very porous to the point that it can absorb 55% its own weight making it a virtual molecular sponge. The pores extend deep into the molecular strata of the zeolite resulting in a high surface area. The depth of these pores allow for continuous effective absorption. Zeolite’s high internal surface area, physical strength and ion exchange properties account for its exceptional absorption capabilities for heavy metals, radioactive elements and a multitude of other harmful elements. Zeolite does not swell as it absorbs. The honeycomb molecular structure allows for the high absorption per weight without losing its structural integrity.
The Zeolite Origin Story - How Zeolite is Formed
Natural, Organic, and Abundant
About Zeolite: Aaron Celestian PhD, Scientist with the NHMLA
The Birth of Clinoptilolite Zeolite
How Natural Clinoptilolite Zeolite was Made

Copyright © greener concrete, LLC - All Rights Reserved
Zeolite Makes the World Better™